SHINVA Endoscopic Imaging System Obtains CE Certification
Recently, the medical
endoscopic imaging system independently developed by Xinhua Surgical
Instruments Co., Ltd., a subsidiary of SHINVA, has successfully obtained CE
certification under the EU Medical Device Regulation (MDR). The company has
been awarded four MDR certificates covering seven product models, laying a
solid foundation for enhancing its competitiveness in the international market.

The medical endoscopic imaging system plays a critical role in SHINVA’s minimally invasive surgical instrument solutions. With its outstanding performance and high quality, the system has already secured orders from countries such as Bangladesh, Chile, and Iraq. This achievement marks a significant step in fostering new growth engines and strengthening SHINVA's global brand presence.
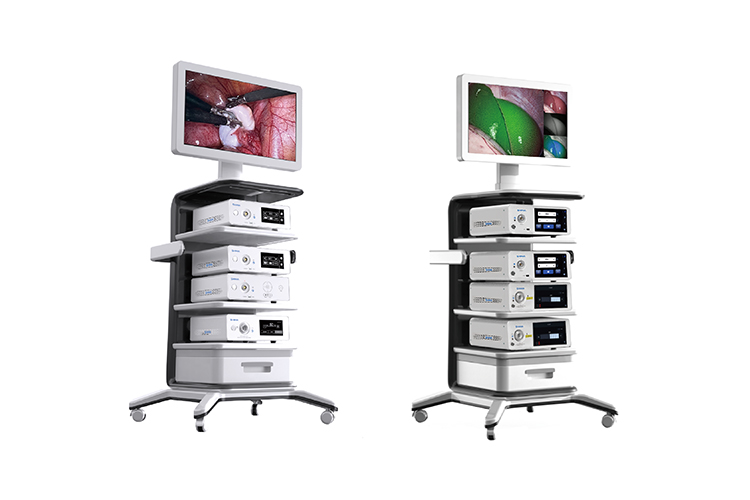
SHINVA has long adhered
to a market- and clinic-driven approach to innovation. The company has
successfully developed a comprehensive range of imaging systems, including HD,
4K, 4K fluorescence, and 3D fluorescence models. These products have gradually
received domestic registration certificates, forming a complete and highly
competitive product portfolio.
Looking ahead, SHINVA
will continue to regard technological innovation as the cornerstone of
development. The company remains committed to market-oriented reforms and will
accelerate the development of new products to further expand into international
markets and earn broader recognition from global customers.